We’re sorry, but we can no longer provide samples of Nanoptek Visible Light Titania. Please contact us for licensing opportunities (February 18, 2025).
Photocatalysts clean water and air like no other agent.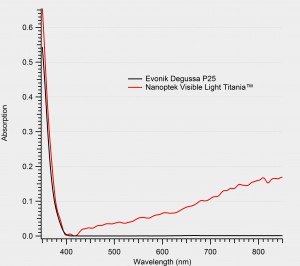
Chlorine disinfects, but it doesn’t break down toxic compounds, and the amount of chlorine used has to be limited and monitored to be safe for human consumption. Germicidal ultraviolet light (with a wavelength of 254 nanometers) is a powerful disinfectant and will also attack some chemical compounds. But it also produces ozone pollution, and must be shielded from people and pets because it causes skin cancer and cataracts. Normal ultraviolet-activated titania photocatalysts do a good job with both disinfection and detoxification, but must be used with artificial ultraviolet sources indoors (although at least the germicidal ultraviolet wavelength is not necessary). Outside, because ultraviolet is less than 5% of sunlight, titania is useful but of limited effectiveness. Further, even the ultraviolet that is absorbed is not used very efficiently by the titania (unless it is our highly efficient UV-Blue™ titania photocatalytic anode), with many of the positive and negative charges recombining before they can work.
We have successfully engineered the bandgap of titania such that it still absorbs high-energy UV, but then begins to absorb in the visible with growing strength even into the near -IR. And yet, tt maintains the chemical inertness and stability of titania.