For decades, hydrogen has been championed as a clean alternative to fossil fuels for transportation and power generation because it is “abundant” and produces only water when converted to power.
Of course, hydrogen in a free state is rare here on earth. Hydrogen has to be produced, either by steam reformation of natural gas (SMR), which consumes a relatively clean fuel and produces 10 tons of carbon dioxide for every ton of hydrogen, or in lesser amounts, by electrolysis of water, an energy-intensive process that also produces carbon dioxide if fossil-fueled generators produce the electricity.
Carbon-free production of hydrogen can be achieved by using electricity from wind or photovoltaic (PV) farms to power proton exchange membrane (PEM) or alkaline electrolyzers. This is being implemented in a number of fledgling projects, from hydrogen refueling stations for fuel cell cars to demonstration homes.
However, the efficiency of electrolyzers, defined as the energy content of the hydrogen produced divided by the total electrical energy required for that production, ranges from at best 70 percent for some alkaline electrolyzers to only 50 percent for many PEM electrolyzers. Since the largest cost driver in hydrogen produced by electrolysis is the cost of electricity, the inefficiency of traditional electrolyzers, in addition to the significant loss of electricity, makes for more expensive hydrogen.
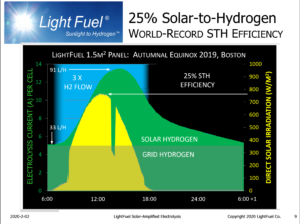
 Lightfuel has a better way to use sunlight for carbon-free production of hydrogen.
Lightfuel has a better way to use sunlight for carbon-free production of hydrogen.
Lightfuel adds Nanoptek’s UV-Blue™ photoanode to the normal anode in an alkaline electrolyzer, placing a shared cathode in between them. When sunlight illuminates the photoanode, holes (positive charges) are generated that split water molecules in the electrolyte into positive H+ and negative OH– ions. The holes and OH– combine to form oxygen gas, while H+ ions migrate to the cathode and combine with the electrons there to form hydrogen gas. AND, the photovoltage so produced adds to the normal anode, increasing its production.